Chronic pain & nociception
Connective tissue layers of the nerve
Peripheral nerves are made up of several hundred nerve fibers (axons). These bundles of axons are surrounded by connective tissue layers to form a fascicle. These fascicles are organised so they are juxtaposed to each other to form a nerve trunk. Sensory fibers: 4 and 10 mm diameter. Motor fibers: 2 and 6mm diameter (Teixeira 2016).
The nerve trunk lies, or is embedded, in a “surrounding tissue bed” comprised of muscles, tendons, fascia, blood vessels, or fat. The paraneurium (or mesoneurium) is composed of connective tissue that attaches the peripheral nerve to its surrounding nerve bed. The paraneurium directs strain along the course of the nerve by guiding the trajectory of peripheral nerves within their beds allowing for low-friction nerve “gliding”. This allows nerve regions that are accustomed to high strain to absorb mechanical loading, as, for instance, parts of the nerve that run over a mobile joint have to be less stiff and more compliant (Foran et al 2018). With elongation of the nerve bed, the paraneurium (or mesoneurium) glides the nerve towards the moving joint (‘convergence’) and consequently, when tension is relieved during joint motion, ‘divergence’ occurs whereby the paraneurium realigns the nerve along the shortened nerve bed by gliding it away from the moving joint (Topp & Boyd 2006).
The three layers of connective tissue that structurally support and regionally nourish the peripheral nerves are:
Epineurium: external layer. The epineurium envelopes several fascicles (bundles of axons, refer perineurium) that together form the nerve trunk (Teixeira 2016). If the nerve contains more than one fascicle, the epineurium may be divided into (i) the external epifascicular epineurium that surrounds the entire nerve trunk; (ii) the internal interfascicular epineurium that separates the nerve fascicles. There is abundant epineurial connective tissue in nerves that contain many fascicles allowing the connective tissue of the epineurium to facilitate the dispersion of a compressive force’s tendency to displace the nerve’s internal contents in transverse and longitudinal directions causing most damage to the axons and myelin where the shear forces are highest at the edges of the compressed zone (Topp & Boyd 2006). The epineurium also resists elongation (Teixeira 2016).
In a slackened position the epineurial collagen fibrils are relaxed with the interfascicular epineurium being loosely attached to the underlying perineurium. This allows one fascicle to slide independently of its adjacent fascicle.
The outermost tissue of the epifascicular epineurium is attached to the paraneurium (or mesoneurium) connective tissue. The density and strength of these attachments differ along the length of a nerve. Where the epifascicular epineurium is less adherent to the paraneurium this loose paraneurium connective tissue contains more adipose tissue to protect the nerve at sites of recurrent compression whilst facilitating transverse and longitudinal gliding of the nerve within the nerve bed. In contrast, the epifascicular epineurium is more tightly adherent to the paraneurium connective tissues where vessels enter or exit the nerve and where the nerve branches. Additionally, there are points at which a nerve may be firmly attached to an anatomical landmark, such as the attachment of the common peroneal nerve near the neck of the fibula (Topp & Boyd 2006).
Collagen fibers from the epineurium forms the outer layer of the dorsal root ganglia capusle (Aliyarbayova et al 2022) and then runs continuous with the outer most meningeal covering the dura mater at the subarachnoid angle (Weerasuriya & Mizisin 2011).
Perineurium (interfascicular connective tissue): middle layer. Made up of a sheath with several layers of perineural flat cells surrounded by a baseline membrane. The perineurium is arranged circumferentially to bundle together axons, Schwann cells, and endoneural components into a nerve fascicle. The perineurium acts as a blood-nerve barrier regulating the endoneuronal environment (Teixeira 2016).
In most peripheral nerves, motor and sensory fibers are intermingled. In general, nerve trunks are grouped into 4-10+ fascicles (bundles of axons surrounded by the perineurium). The perineurium continuously and individually covers these fascicles, that collectively form the nerve trunk, all the way down to the nerve terminal (Teixeira 2016).
However, axons do not remain in the same fascicle throughout their length and can move by means of connective tissue layers to other fascicles. The interchange of axons between fascicles help to minimise functional deficits following partial injury to the nerve, but, can also result in a wide distribution of macrophages cleaning up the debris from axons undergoing Wallerian degeneration after injury (Topp & Boyd 2006).
The perineurium divides into (i) the superficial layer of the perineurium forms the inner layer of the DRG capsule (Aliyarbayova et al 2022) and attaches to the middle meningeal covering, the arachnoid mater, at the subarachnoid angle. The arachnoid mater at this level forms the outer layer of the root sheath which then runs continuous with the pia mater at its emergence from the spinal cord; (ii) the inner layer of the perineurium continues to become the inner layer of the root sheath (Weerasuriya & Mizisin 2011) .
Endoneurium (intrafascicular connective tissue/Henle’s sheath): inner layer. Made up of connective tissue that individually surrounds intrafascicular Schwann cells-axon unit and fine fluid and fibrillar material as well as sheltering blood capillaries. The collagen fibrils of the endoneurium are closely packed around each Schwann cells-axon unit to form the supporting wall of the endoneurial tube. As the endoneurial tube encases the axon, its Schwann cell sheath, and the myelin (Menovsky 1999) they guide axonal regeneration (Teixeira 2016).
The axons of the peripheral nerve trunk have elasticity and a corrugated pathway so that they can be elongated up to 50% of their length before tension is directly transmitted to nerve tissue. Nerve roots have less connective tissue and are less corrugated than individual axons making them more vulnerable to mechanical distortion (Teixeira 2016).
Where the dorsal and ventral root attaches to the spinal cord the endoneurium of the spinal roots is continuous with the subarachnoid space providing continuity between CSF and endoneurial fluid (Weerasuriya & Mizisin 2011). The arachnoid mater that forms the nerve sheath of the intrathecal spinal roots is elastic, whereas the continuing epineurium that ensheathes the spinal nerves and more distal nerve trunks is relatively inelastic (refer ‘epineurium’). This point of stress between the elastic nerve root sheath and inelastic covering of the spinal nerves and trunks may create endoneurial inflammatory oedema stretching the relatively inelastic epi-perineurium beyond the limit of its compliance. This results in constriction of the transperineurial vessels accompanied by endoneurial ischaemia and nerve conduction failure (Berciano 2018).
The different type of Schwann Cells (a glial cell) include:
Myelinating Schwann cells. The plasma membrane of a Schwann cell is wrapped tightly multiple times around the axon forming the myelin sheath of each myelinated axon. Thus, a single Schwann cell envelops a single myelinated axon producing non-conductive myelin in large diameter myelinated axons which increases conduction speed. Each Schwann cell covers 300 to 2000mm of the axon length, being longer in larger axons.
The uninsulated 1mm gaps in the myelin sheath, the points of separation between the myelinating Schwann cells, are called nodes of Ranvier. The nodes of Ranvier are enriched with ion channels that enables them to exchange ions to regenerate action potentials. Unmyelinated axons are enveloped by Schwann cell cytoplasm and plasma membrane but do not have multiple wrappings of Schwann cell plasma membrane. A single Schwann cell may envelop several unmyelinated axons (Topp & Boyd 2006).
Myelinating Schwann cells can be triggered by a peripheral nerve injury to change function and aid regeneration by increasing the production of growth factors. However, as most Schwann cells are axonal sheaths located outside the DRG the specific changes that occur post-injury in the Schwann cells to effect nociception in the DRG is questionable (Martin et al 2019).
Satellite glial cells (nonmyelin-forming Schwann Cells). An accumulation of cell bodies from thousands of somatic, sympathetic, and parasympathetic sensory neurones, (up to 70,000) are located in the DRG, trigeminal ganglia and other ganglia associated with the cranial nerves. The cell bodies are surrounded by envelopes of satellite glial cells (SGCs) that form adhesive, tight, and gap junctions, are enriched for cytokine and interleukin signalling expressing proinflammatory molecules, buffers potassium concentrations in the ganglia and regulates gliosecretion in astrocytes after injury. Therefore, SGCs modify the microenvironment of neurons by regulating the uptake and release of molecules that supply nutrients to the surrounding neurons having a trophic function, influence synaptic transmission and have some structural function.
SGCs proliferate and alter after a peripheral nerve injury (Martin et al 2019):
(i). Their adhesive, tight gap junction temporarily becomes bigger.
(ii). The number of activated SGCs connected with SGCs of other neurones increases forming a ‘glial cohesion’ whereby more cell bodies in the ganglia share a common glial sheath; (iii) the SGCs loose their potassium regulatory functions elevating potassium concentrations in the ganglia, which, within itself, and by down-regulating the SGC removal of glutamate from the cell bodies (as well as increasing importation of glutamate) increases neuronal excitability (Krock et al 2023). Glutamate is the primary nocicpetive transmitter in the DRG (Haberberger et al 2019).
(iv) the release inflammatory transmitters induces chemical signals which generate electrical signals that get transmited to the DRG or trigeminal ganglion e.g. pain —> increase nerve impulses to DRG —> SGCs and neurones release large quantities of ATP (& proinflammatory cytokines) —> increase intracellular calcium concentration —> increase neuronal excitability, sensitivity and activation of SGCs (refer ‘Mitochondria & sensory processing’) (Krock et al 2023).
This makes SCGs central to the inflammatory process, and hence, increased DRG neuronal cell death (Martin et al 2019) and by how this influences neuronal excitability, the consequential development and maintenance of chronic pain (Andreeva et al 2022) (refer ‘Compression (or systemic inflammation) —> neuroinflammation —> neural oedema —> impaired axoplasmic flow. Neuroinflammation & impaired axoplasmic flow —> axonal mechanosensitivity —> peripheral sensitisation —> central sensitisation’). As DRG size increases from C1-8 and from L1-5 but decreases from S1 to S4 (with a very large ganglion at S1) could the correlation between DRG size and number of neurones contained within the DRG (Haberberger et al 2019) reflect severity of symptomology in resposne to pathological changes?
The central role of SGCs to the inflammatory process in the DRG can be seen in fibromyalgia. The association between IgG binding to SGCs (the autoantibody ‘anti-SGC IgG’) in the DRG and nociceptor hyperactivity and reduced intraepidermal nerve fiber density has been documented in fibromyalgia patients (Krock et al 2023). These IgGs binding to SCGs in the DRG produce spontaneous pain by, potentially, increasing spontaneous activity in, and sensitising nociceptive afferents. Even though these IgGs don’t bind to any other cell in the spinal cord or brain and are not involved in central mechanisms of evoked pain that can be seen in fibromyalgia patients, high levels of these ‘anti-SCG IgG’ autoantibodies are associated with low levels of certain metabolites in the thalamus and rostral anterior cingulate cortex that is associated with fibromyalgia disease severity (Fanton et al 2023).
In sensory nerves even though there are five thousand to nine thousand myelinated fibers (varies to individuals), there are two to six times more unmyelinated fibers. Schwann Cells-axon unit diameters are 0.5- 3.0mm < 1.5mm, increase myelin —> increase Schwann Cells-axon unit diameter (Teixeira 2016).
Group C-fibers are unmyelinated and have low conduction speed (Group A and B are myelinated and have high conduction speed). Schwann Cells bundle these C-fiber axons close together but stops them touching by squeezing the cytoplasm between the axons (Teixeira 2016).
Vascular supply to the nerve
Blood supply to the dorsal root ganglia
The DRG doesn’t just contain the neuronal cell bodies of primary sensory neurones, their axonal hillock, initial segment (enveloped by SCGs) and its continuing peripheral axon (enveloped by the meninges including the dura mater that is continuous with ganglion sheath), but small blood vessels. These blood vessels deliver blood and oxygen to satisfy the extensive energy demand of sensory neurones that have long processes, and so have a high-energy demand that is critical for maintaining the production and transport of receptors, ion channels, cytoskeletal and transport proteins (Haberberger 2019).
This extensive network of arterioles and capillaries that interface with the sensory neurones and blood vessels in DRG is unique making the DRGs highly vascularised. The fenestarted capillaries allow large quantities of blood borne molecules circulating in the vascular system to directly enter the DRG and interact with neuronal cells and non-neuronal cells including the immune cells that are contained within DRG that consist of macrophages (one of the key cells that modulate pain signalling), T-lymphocytes and B-lymphocytes (Haberberger 2019). The downside to these fenestrated capillaries allowing large quantities of blood molecules to enter and irrigate DRG is that antigens, infecting agents or immune cells can easily enter the DRG such as the Herpes virus and IgGs which bind to SGCs in the DRG (the autoantibody ‘anti-SGC IgG’) (Krock et al 2023) producing pain, rather than evoked pain in fibromyalgia (Fanton et al 2023).
This vascular organisation allows for an efficient exchange of nutrients, gases, and metabolites in the DRG and has two interconnected deep and superficial arterial plexuses that has a significantly higher perfusion in women compared to men (Haberberger 2019).
Blood supply to the nerve trunk
Nerve trunks are perfused by blood vessels originating from the collaterals of adjacent arteries. Vascular and inflammatory responses in the nerve accompany fibrotic tissue in the nerve’s surrounding connective tissue (Kitamura et al 1995).
The peripheral nerves have an extrinsic and intrinsic vascular supply:
Extrinsic vascular system: includes coiled segmental regional vessels that run in the loose "adventitia" or along the paraneurium (or mesoneurium) surrounding the nerve. The looseness of this “adventitia” or paraneurium allows the nerve trunk considerable mobility in its bed (Kitamura et al 1995).
Intrinsic vascular system: comprises the vasonervorum. It includes numerous vascular plexa in the epineurium, perineurium, and endoneurium (Kitamura et al 1995). After perforating the epineurium, the arteries divide into epineurial arterioles that form an anastomotic network that run within the epifascicular and interfascicular epineurium. Perforating arterioles cross the perineurium carrying with them a short sleeve of perineurial cells into the fascicle. Perineurial arterioles have poorly developed smooth muscles which limits their ability to regulate intrafascicular blood flow. Within the intrafascicular endoneurium, arterioles become large-diameter capillaries that form wide anastomotic longitudinal chains allowing blood to flow in either direction. Although the endothelial cells of endoneurial capillaries are connected by tight junctions to form a tight blood-nerve barrier (Topp & Boyd 2006), which selectively regulates the transfer of circulating substances inside the endoneurium (Teixeira 2016), due to the absence of astrocytes, this physical barrier is considered to be looser than the BBB in the CNS (Li et al 2023).
Vascular supply and fibrosis of the nerve
When the peripheral nerves are stretched, the surrounding blood vessels are extended, resulting in decreased blood flow within the nerve bundle. How this effects the extrinsic and intrinsic blood flow varies (Kitamura et al 1995):
Extrinsic system: blood flow decreases sharply in the presence of relatively small amount of traction and then decreases in a slow linear fashion with further increases in traction.
Intrinsic system: on the other hand, the blood flow through the intrinsic system shows a slower linear decrease in response to traction.
The difference in blood flow between the two systems can be explained by the biomechanics of the nerve in response to stretch. If stretching the nerve stretches the blood vessels then stretching the blood vessels will result in less circulation. In such a case the area stretched the least and has the better circulation should redirect blood to the area that is stretched the most and has the worst circulation (Kitamura et al 1995).
When a nerve is stretched blood is redirected from the less elongated portion i.e. the extrinsic system (paraneurium), as it flows through the loose “adventitia”, to the more elongated portion i.e. the intrinsic system (epi-, peri- & endoneurium), that gets pulled tighter (Kitamura et al 1995).
The sharp decrease in the blood flow through the extrinsic system can lead to local elevation of vascular permeability and oedema in the internal system. With repeated stretching over a two week span this has been shown to lead to hypertrophic connective tissue in the paraneurium and nerve bed and vascularisation of the epineurium and the surrounding tissue (Kitamura et al 1995). Could this cause a cascade that results in a further decrease movement by paraneural fibrosis, and in turn vascular-fibrotic changes in the nerve?
In response to peripheral nerve injury, as opposed to repeated stretch, fibrotic cells enveloping blood vessels in all three connective tissue layers of the nerve compartmentalise the endoneurium into many small fascicles. This can result in a persistent hypoxic state of the nerve, including microvascular dysfunction, endoneurial fibrosis, and increased metabolic loads (Lim et al 2015).
Could epiperineural fibrosis, causing a dynamic intraneural compression of the intact axons and their bundles, result in nociceptive sensitisation (refer ‘Intermittent (dynamic) partial compressive nerve lesion as an essential cause of persistent sensitisation and pain’ (Macionis 2023)? Could also endoneurial fibrosis restrict the internal mobility of the nerve bundle again creating a sensitisation? This type of fibrosis is seen around the brachial plexus during operations on patients with traumatic thoracic outlet syndrome (Chuang et al 2016).
Nervi nervorum
Nerve trunks are mechanosensitive because their connective tissue has afferents that participate in mechanoreception. Peripheral nerves are innervated by the nervi vasorum (that innervate the epineural blood vessels) and the nervi nervorum. The nervi nervorum are made up of small (unmyelinated or thin myelinated) fibers in the epineurium, perineurium and endoneurium (including encapsulated Pacini corpuscles in the endoneurium) (Teixeira 2016).
There are four distinct regions of peri- and non-perivascular innervation of the nerve trunks (Teixeira 2016):
Perivascular innervation of extraneural arteries and veins.
Perivascular innervation of intraneural blood vessels: vasa nervorum.
Non-perivascular fibers, the nervi nervorum, originates from the nerve trunk and perivascular plexus (vasa nervorum) and is distributed by epineural, perineural and endoneural connective tissues.
Intrafascicular fibers has a major adrenergic component, particularly in the sciatic nerve.
Anatomical peculiarities of the nervi nervorum and vaso nervorum makes them vulnerable to stretch and damage. Injury to the nervi nervorum can result in nerve sprouting and the development of neuromas. The result can be neuronal hyperreactivity (Teixeira 2016), and as a result of this C-fibers activity the release of angiogenic inflammatory substances in the form of increase in peripheral blood flow attributable to the release of vasoactive neurotransmitters from peripheral nociceptor terminals (Wu et al 2002) targeted at the vasa nervorum. These inflammatory substances can causes neurogenic inflammation and regional oedema precipitating a vicious compression-inflammation cycle (Teixeira 2016). Therefore, the nervi nervorum is both nociceptive and nocifensive as it responds to damaging stimuli by contributing to local inflammation in order to help defend and maintain the nerve’s local environment (Bove 2008). The ectopic impulses generated by the nervi nervorum, as a consequence of this injury cycle, can give local as well as referred pain (Teixeira 2016).
Neurogenic inflammation generated by nervi nervorum propagates mechanosensitivity along the nerve trunk distal from the injury site. Nervi nervorum sensitisation accounts for almost 50% of presentations of pain and paraesthesia that does not correspond to regions of the affected nerve root (Teixeira 2016).
However, when entrapment neuropathies lead to the structural degeneration (as opposed to sensitisation) of the small unmyelinated C-fibers that form the nerve nervorum there is decreased firing on neurodynamic testing. This may account for the >54% of patients with CTS having negative neurodynamic tests and approximately 58% of patients with lumbosacral radiculopathy have a negative SLR (Baselgia et al 2017). It should also be remembered that pathologies casuing demyelination of the nerve, whilst causing spurious activity in axons, does not necessarily affect the nociceptors as they are not myelinated (Bove 2008). As well as nocicepetive function tested on neurodynamic testing C-fibers can also be tested by pin prick and hot/cold sensation.
Biomechanics of the nerve
Elongation of the nerve
On initial stretch to a resting nerve, the nerve lengthens markedly so that the wavy connective tissue and axons in the endoneurial core straightens out. As further stretch is applied the nerve elongates (‘creeps’) at a steady rate (Topp & Boyd 2006).
Elongation of a nerve reduces it cross-sectional area, known as transverse contraction. This results in an increased pressure in the endoneurial core which, in turn, resists further transverse contraction and contributes to the stiffness of the nerve when under stretch (< centre of the elongated segment). The interface between the surrounding inner most layer of the perineurium, and the pressurised endoneurial core which it constrains, provides some minimal resistance to elongation of the nerve (Topp & Boyd 2006).
With elongation of the nerve bed, the paraneurium (or mesoneurium) glides the nerve towards the moving joint (‘convergence’) (refer ‘Connective tissue layers of the nerve’). For example, elbow extension elongates the median nerve as it aligns itself along the lengthened nerve bed. The paraneurium does this by gliding the nerve segment in the arm distally towards the elbow and the nerve segment in the forearm proximally towards the elbow. Consequently, when tension is relieved during joint motion ‘divergence’ occurs whereby, for example, during elbow flexion the median nerve slackens back off as the paraneurium realigns the nerve along the shortened nerve bed by gliding it away from the moving joint. With limb movement, nerve excursion occurs first in the nerve segment immediately adjacent to the moving joint, where the magnitude of excursion is greatest, and then as the limb continues to move, excursion occurs at nerve segments that are progressively more distant from the moving joint (Topp & Boyd 2006).
With increasing stretch stages of structural separation occurs: (i) in the interface between the perineurim and endoneurial compartment; (ii) between the axons and connective tissues in the endoneurial core; (iii) in the cells and connective tissues of the perineurial and epineurial sheath. Therefore, damage to axons in the endoneurial core may occur long before visible damage to the epineurium (Topp & Boyd 2006).
Factors influencing nerve stiffness
The paraneurium directs strain along the course of the nerve by guiding the trajectory of peripheral nerves within their beds allowing for low-friction nerve “gliding”. This allows nerve regions that are accustomed to high strain (i.e. areas of the nerve that are less stiff and highly compliant) to absorb this mechanical loading (Foran et al 2018). Factors influencing nerve stiffness are (Topp & Boyd 2006):
There is greater nerve compliance and less stiffness in nerve segments that cross joints, rather than in segments that do not cross joints.
There is less compliance and greater stiffness at points where the nerve is firmly attached to an anatomical structures, such as the attachment of the common peroneal nerve near the neck of the fibula.
Severing nerve branches or vessels results in increased compliance and decreased stiffness of the nerve. The epineurium is more tightly adherent to the paraneural connective tissues where vessels enter or exit the nerve and where the nerve branches.
The nerve shows less compliance and greater stiffness when the nerve is elongated rapidly rather than slowly. However, as this stretch is maintained there is a reduction in the tension in the nerve. A majority of this relaxation occurs in the first 20 minutes of fixed elongation as the nerve tissue elongates gradually (“creeps”) under the sustained load in order to provide protection in postures whereby the nerves are lengthened under prolonged tensile strain.
Chronic pain: nociception
Chronic pain, i.e. pain that lasts more than three months, is traditionally thought to be principally due to central sensitisation, which is driven by neuroinflammation in many chronic pain conditions. Although central mechanisms of sensitisation are important, they cannot completely account for such chronic pain features as chronicity, spontaneity, and, most importantly, individual variability in occurrence. The evidence supporting central sensitisation (autonomous continuing input-independent pain) as a source of chronic pain is relatively scanty and is outweighed by contrary evidence (Macionis 2023).
So where can nociceptive input come from when there is no obvious pathology? Could the answer be because the paraneural tissue damage, i.e. noxious input, resulting in stimulation of nociceptive fibers experienced in acute pain, persist and so sensitise the nociceptive fibers to become chronic pain? Peripheral nerve injury, although associated with neuropathic pain, is recognised as a cause of chronic pain, e.g. CRPS type I, fibromyalgia, and numerous small fiber neuropathy (SFN) conditions (Macionis 2023).
Three types of pain may manifest when a peripheral nerve is damaged (Teixeira 2016):
Pain at the site of nerve trunk injury. Described as stabbing or tender. This is attributed to increased activity of abnormal nociceptors that have been chemically or mechanically sensitised (Teixeira 2016). After a few days of inflammation axons become capable of generating autonomous (ectopic) action potentials, without stimulation from their terminal receptive fields. For instance, instead of generating an action potential from touching the skin an inflamed axon will just spontaneously fire off an action potential. The peak of these ectopic impulses occur four to seven days after the initial insult, and then decreases to insignificance or heals within two months (Bove 2008).
Disestesic and piercing pain. Described as burning, smarting or tingling or electricity. Disestesic pain is a consequence of regeneration of nociceptors which become abnormally excitable from abnormal activation of nociceptive afferents in the nervi nervorum.
Paroxysms. Described as a sudden attack of shock, stabbing, jumping or a deep pain following the nerve trunk and can be accompanied by allodynia. Symptoms are localised in the distribution of a sensory, or mixed nerve, and correspond to areas of sensory deficit and allodynia. These signs and symptoms are attributed to hyperactivity of mechanically and chemically sensitised nociceptors of the nervi nervorum whereby the pain is worsened with movement, stretching or palpation of the nerve trunk.
Compression (or systemic inflammation) —> neuroinflammation —> neural oedema —> impaired axoplasmic flow. Neuroinflammation & impaired axoplasmic flow —> axonal mechanosensitivity —> peripheral sensitisation —> central sensitisation
The peripheral nerves are very susceptible to overload, nerve function can be disturbed by pressures as low as 30 millimeters of mercury with ongoing neuronal activity developing after three weeks of repetitive overuse (Macionis 2023).
Paraneural tissue injury and inflammation < peripheral nerve lesion (e.g. nerve compression) —> neural oedema —> impairs axoplasmic transport. Neuroinflammation and impaired axoplasmic transport leads to peripheral sensitisation of afferent fibers (A- and C-type) causing hyperexcitability and consequential hyperactivity of the PNS (Macionis 2023). This is seen, for example, in peripheral nerve trunk pain, where the small fibers of the nociceptive nervi nervorum become sensitised (Teixeira 2016). As well as sensitising afferent nociceptive nerves these inflammatory mediators also trigger damage to sympathetic nerve fibers in the joints contributing to inflammatory arthritic pain (Silva et al 2022).
This axonal mechanosensitivity may explain why the most prevalent anatomic sites of chronic pain are those with high degrees of mobility, are subject to mechanical overload and are tightly interconnected with the spinal plexuses e.g. the extremities and lower back (Macionis 2023). This is because with movement, nerve excursion in these mechanosensitive nerves occurs first in the nerve segment immediately adjacent to the moving joint, where the magnitude of excursion is greatest, and then as the joint continues to move, excursion occurs at nerve segments that are progressively more distant from the moving joint (Topp & Boyd 2006). Endoneurial oedema, which can cause nerve conduction block, may quickly develop because of repetitive compression at these mobile sites where these mechanosensitive nerves have to be more compliant and less stiff, and maybe an ectopic cause of repetitive strain injury (e.g. nonspecific arm pain) (Macionis 2023). This may contribute to C2 DRG symptomology as the unusual relationship of the DRG being posterior to the C1-2 facet joint (Haberberger et al 2019), and the encasing of the C2 DRG, either within the posterior atlanto-axial ligament, or within an investing fascia that holds the C2 DRG, roots and spinal nerves against the capsule of the C1-2 facet joint can produce (Bogduk 1981) permits substantial mobility of a mechanosensitive C2 DRG.
Whilst axonal mechanosensitivity can be induced by atraumatic non-inflammatory disruption to axoplasmic flow, spontaneous, ongoing nociceptor activity can only occur with a focal neuritis (neuroinflammation). However, in reality neuroinflammation is likely to accompany all nerve injuries (Macionis 2023).
Nociceptive sensitisation, from the neuroinflammatory response to a neuritis (arising from both neural and non-neural tissue), and ion channel mutations causing a reduced activation threshold of ion channels, creates ongoing (spontaneous) activity and mechanical sensitivity of primary sensory fibers. This can account for tissue hypersensitivity in the form of disproportionate pain to noxious stimuli (hyperalgesia) and to non-noxious stimuli (allodynia) (Macionis 2023).
These ectopic neural impulses (i.e. action potentials not arising normally from nerve ending receptors) has traditionally been classified as being generated from nerve injuries stimulating sensory fibers and radiculopathies stimulating neuron cell bodies. However, recently, it has been found that these ectopic, spontaneous impulses in chronic nerve constriction is generated by the initial segment.
Normally sensory DRG neurones generate action potentials at the peripheral ending and then the axon initial segment and axon hillock, that are located in the DRG, determines the passage of action potentials to the central terminals of the sensory neurones in the dorsal horn (Haberberger et al 2023). However, nerve injury-induced DRG inflammatory changes, and consequential activation of SCGs, produces sensory neuron hyperexcitability (Macionis 2023) and spontaneous generation of action potentials in the sensory nerve at its axon initial segment (Haberberger et al 2023).
As well as containing the cell bodies and the axon initial segment encased by SCGs, that extends to a peripheral axon whose epineurium is encased by the meninges, including a thick layer of dura mater that is continuous with ganglion sheath, the DRG also contains an extensive network of arterioles and capillaries. These capillaries are fenestrated so that many inflammatory molecules such as macrophages (one of the key cells that modulate pain signalling), T-lymphocytes and B-lymphocytes can directly enter the DRG and interact with neuronal cells and non-neuronal cells (Haberberger et al 2019). Whilst the DRG contains low numbers of nerve endings from neurones that originate from outside the DRG, in response to nerve injury these numbers increase causing a sprouting of sympathetic and sensory fibres into the DRG (Haberberger et al 2023) that may potentially complicate the inflammatory changes and generation of ectopic impulses in the DRG.
Whilst acute inflammation is required to clear the cellular debris and improve the environment for regeneration after two months of chronic inflammatory changes from a peripheral nerve injury up to 35–40% of DRG neurones die (Martin et al 2019). The hyperactivity and spontaneous activity in the surviving afferent DRG neurones generate spontaneous, ectopic impulses (Macionis 2023).
Either from chronic inflammatory changes or cellular death of the DRG neurones the ongoing ectopic activity and axonal mechanosensitivity of afferent fibers (both of the A- and C-type) is induced by persistent intermittent (dynamic) partial proximal paraneural and intraneural compression (e.g. myofascial tension) —> peripheral nerve damage. This intermittent, partial injury to the paraneural tissues producing focal neuroinflammation results in a loss of DRG neurones and DRG neuron body sensitisation accounting for ongoing activity in nociceptive neurons (nococieptive sensitisation) (Macionis 2023).
It is crucial that the injury to the paraneural tissues is intermittent and partial as more extreme degrees of nerve compression results in axonal death of neural tissue which reduces nerve function (e.g. loss of motor power and anaesthesia), and creates a discontinuity between the axon and the nervi nervorum reducing its mechanosensitivity (Macionis 2023).
This DRG neuron hyperexcitability and consequential hyperactivity, in turn, triggers a hyperexcitability and consequential hyperactivity of the CNS. When these hyperexcitable and hyperactive action potentials in the CNS are processed in the brain cortex as persistent pain, and an abnormal extraterritorial hypersensitivity of non-lesioned tissue ensues, the patients has progressed to a state of central sensitisation (Macionis 2023).
Intermittent (dynamic) partial compressive nerve lesion as an essential cause of persistent sensitisation and pain
Extreme degree of distal nerve compression, in contrast to that of a moderate grade, does not result in peripheral tissue hypersensitivity. This is because extreme degrees of nerve compression triggers axonal degeneration resulting in a discontinuity between the axon and the nervi nervorum, a complete resolution of distal focal inflammation and the subsiding of ectopic impulses (Macionis 2023).
Inflammatory changes in the DRG and spinal cord are more pronounced after chronic nerve constriction, rather than a complete transection of the nerve. Therefore, it is persistent intermittent (dynamic) partial proximal peripheral nerve damage (e.g. myofascial tension) with its resultant continuing, chronic, focal neuroinflammation that causes a consequential hyperexcitability of the DRGn (Macionis 2023).
Although healed (fibrotic) neural lesions are physiologically silent, and so consequently cannot provide nociceptive input, scarred paraneural tissue can cause intermittent neural damage by restricting mobility of nerves. Via this mechanism, intraneural fibrosis of peripheral nerves may also result in dynamic intraneural compression of intact axons and their fasicicles (Macionis 2023) (refer to ‘vascular supply of the nerve; vasculat supply and fibrosis of the nerve’).
Intact axons and bundles of axons are relatively mobile; axons in the peripheral nerve trunk are elastic and have a corrugated pathway so they can elongate up to 50% of their length before tension is directly transmitted to nerve tissue (Teixeira 2016) and loose connective tissue allows movement of axons and fascicles in respect to each other. Therefore, any epiperineural scarring surrounding these healthy, mobile axons and fascicles may lead to an internal, dynamic, entrapment during motion. Whilst this type of dynamic intraneural nerve compression may produce only subtle nociceptive input, it may be sufficient to maintain central sensitisation via long-term potentiation (Macionis 2023). This impairment of the nociceptive inhibitory system being associated with central sensitisation is illustrated in migraine patients who exhibit more distal, broader, sensitivity in the tibialis anterior and median nerve (Del-Blanco-Muñiz et al 2023).
Nerve compression may intermittently be produced not only by neuromuscular but also by neurovascular mechanisms as seen, for example, in trigeminal neuralgia. This neurovascular mechanism, involving reflexive vasodilation, vasoconstriction and transient oedema, may be responsible for both intraneural and extraneural compression. In immobile areas, such as the skull, dynamic neural compression may only be produced by neurovascular mechanisms (Macionis 2023). However, a double crush syndrome involving mechanosensitivity of the trigeminal nerve (refer ‘double crush syndrome and sensitisation’) involving mechanosensitivity of the trigeminal nerve as the C2 DRG is unique in being posterior to the C1-2 facet joint (Haberberger et al 2019), whereby the encasing of the C2 DRG, either within the posterior atlanto-axial ligament, or within an investing fascia that holds the C2 DRG, roots and spinal nerves against the capsule of the C1-2 facet joint (Bogduk 1981) permits substantial mobility of a mechanosensitive C2 DRG.
However, it should be remembered that even in immobile areas sensory nerves can still be mobile because of the dynamic structures they traverse or terminate in e.g. the trigeminal branches glide because of facial muscle activity. Therefore, even in these cases both para- and intra-neural fibrosis can still result in intermittent nerve compression (Macionis 2023).
Figure one: Chronic pain cycle. (Macionis V. 2023. Chronic pain and local pain in usually painless conditions including neuroma may be due to compressive proximal neural lesion).
Double crush syndrome and sensitisation
Double crush syndrome (Macionis 2023):
(i). Primary compression —> physically impairs intra-axonal circulation and neuroinflammation —> neural oedema —> impair axoplasmic transport —> activates the DRGn (Macionis 2023). Impairing axoplasmic flow reduces the axonal transport of mitochondria (refer ‘figure three’) impairing nerve structure and function, promoting inflammation, pain, excitability, demyelination and effects the release of neurotransmittors (Silva et al 2022).
(ii). Secondary lesions. Secondary lesions do not necessarily have to be compressive but may entail genetic mechanisms or systemic neuroinflammation producing neural oedema and impairing axoplasmic transport (Macionis 2023).
Because the site of primary compression impairs axoplasmic transport and causes swelling in the nerve, this makes it more prone to secondary nerve compression and causes axonal mechanosensitivity along the whole course of the nerve. Therefore a secondary lesion to this already swollen and sensitised nerve not only disproportionately activates the DRGn to a sum greater than the insult of each lesion individually but makes the nerve trunk more susceptible to further damage and produces disproportionate axonal degeneration and neurological deficits (Macionis 2023).
Just as a disruption of axoplasmic transport facilitates secondary nerve compression and causes axonal mechanosensitivity, neuroinflammation induces both axonal mechanosensitivity and ongoing nociceptor activity. However, in reality neuroinflammation is likely to accompany all nerve injuries (Macionis 2023).
Distal vs. proximal neural lesions
Distal peripheral nerve lesions lead to proximal neural involvement in the form of DRGn ectopic hyperexcitability, DRG fibrosis and neuronal death in the dorsal horn. The more proximal the injury the more detrimental this process is (Macionis 2023).
Increases in axoplasmic calcium following axonal damage triggers Wallerian degeneration. Retrograde signals sent from the injury site to the DRG cell bodies increases production of the growth and survival factors that aid neuronal regeneration. But as well as initiating the production and transportation of trophic functions to aid neuronal regeneration these signals also contribute to inflammatory changes. This results in DRG neuronal cell death and nociceptive changes producing hyperactive, spontaneous ectopic impulses in the surviving DRG neurones and eventually spinal cord central sensitisation (Martin et al 2019). As Wallerian degeneration not only occurs locally, but also distally from the injury site, potentially extending over the length of a nerve, a level of greater sensitisation occurs not only from its ‘spreading’ deymyelination effect but also by inducing spontaneous activity in the neighbouring uninjured C-fibers in the nervi nervorum (Macionis 2023). For instance, lesions to the L5 ventral (motor) root fibers produce inflammatory products, that spill over on to, and in turn produce spontaneous activity in adjacent uninjured L4 nocicpetive C-fibers (and L5 nociceptive afferents) producing mechanosensitivity in the form of mechanical hyperalgesia (Wu et al 2002). This sensitisation is heightened further in the case of proximal, rather than distal nerve lesions (Macionis 2023).
Compressive neuropathic aetiology of generalised chronic pain: the vicious cycle
Chronic pain may be caused by compensatory overuse-related compressive proximal neural lesions (Macionis 2023).
Initial posttraumatic pain —> postural protection —> compensatory musculoskeletal weakness and overuse (Macionis 2023).
Weakened muscles are naturally more susceptible to overuse and myofascial tension which can result in proximal nerve compression (Macionis 2023). This can be seen in migraine patients where decreased strength of the longus colli muscle leads to diminished neck flexion strength and altered head positioning predisposing to sensitisation of nerves in the upper cervical spine (Del-Blanco-Muñiz et al 2023).
It is damage to the motor fibers that mediate the reciprocity between loss of muscle strength and compressive proximal neural lesions. Wrong posture, ligament laxity and muscle shortening may also contribute to development of compressive proximal neural lesions as seen, for example, in the biomechanical pathogenesis of TOS (Macionis 2023).
Primary CNS lesions that activate antidromic activation of the DRGn (action potential traveling in the opposite physiological direction), peripheral tissue damage or radiculopathy —> hyperexcitability of DRG neurons —> proximal reflexive muscle spasm and myofascial tightness, which, due to its compression at anatomically narrow spaces (compressive proximal neural lesions) —> production (or aggravation) of focal neuroinflammation of the proximal nerve trunk at the compression site. This compression maintains this cycle by inducing hyperexcitability of the DRG neurons (Macionis 2023).
It is essential however that the nerve pain be of a persistent proximal neural lesion of intermittent character. Proximal for its potent effects on the CNS and intermittent so that it doesn’t kill off the nocicepetive fibers (Macionis 2023).
This cycle may be perpetuated by: (i) reciprocity between compressive proximal neural lesion caused by motor fiber dysfunction and muscle weakness leading to muscle imbalance and compensatory overuse; (ii) sensitisation as sensitised nerve trunks become more susceptible to further damage. Therefore, a single-site compressive proximal neural lesion, by sensitising the primary nerve trunk, then the secondary nerve trunks that are associated with the primary nerve trunks DRG, and finally, through sensitisation of the CNS, a sensitisation of peripheral nerves all over the body, may result in multiple-sites of compressive proximal neural lesions. This may be another explanation of bilateral widespread chronic pain (Macionis 2023).
Why does occurrence of chronic pain vary from patient to patient?
Susceptibility to pain may represent a predisposition to compressive proximal neural lesions from (Macionis 2023):
Age: gradual decline of muscle strength.
Women: relatively high susceptibility of their musculoskeletal system to overload and injury leads to consequential compressive proximal neural lesions.
Genetics. For example, the growth and extracellular matrix architecture and paraneural anatomical peculiarities (e.g. the dimensions of the anatomical tunnels the nerves travel through) (Wiberg et al 2019).
Neurophysiology.
Explanation of trigeminal neuralgia, temporomandibular pain, and other orofacial pain
Because of relative shortness of the trigeminal nerve, its lesion at any level can be regarded as a peripheral nerve lesion. In trigeminal neuralgia, the trigeminal nerve is compressed (most often via a neurovascular mechanism) proximal to its ganglion, while in TMD the more mobile V3 part of the nerve is affected (Macionis 2023).
This creates a predisposition for motion-maintained intermittent compressive proximal neural lesions of V3 at:
Its exit from the skull (foramen ovale, greater wing of sphenoid) which is very close to the trigeminal ganglion (Macionis 2023).
Superior head of lateral pterygoid: middle deep temporal nerve passes through this muscle (Kwak et al 2003).
Fusion between the deep temporal and lateral pterygoid fascia: buccal nerve (V3) and anterior deep temporal branches of trigeminal nerve (V3). The buccal nerve (V3) after passing within the fused lateral pterygoid and deep temporalis fascia lies against, or imbeds within, the most medial fibers of the deep part of the temporalis (Gaughran 1957). The anterior deep temporal branches of trigeminal nerve (V3) run in the temporalis muscle.
Interpterygoid fascia: the auriculotemoporal nerve (V3) pierces the interpterygoid fascia (Barker and Davies 1972).
Lesions of a single peripheral division of the trigeminus (e.g. V3) inducing inflammation in the trigeminal ganglion (Macionis 2023), and, excitation due to the inflammatory spill over from Wallerian degeneration on to uninjured V1 and V2 branches, leads to sensitisation and a spreading of symptomolgy into the V1 and V2 nerves (Wu et al 2002).
Explanation of low back pain
A blockage of normal movement in back pain patients, as opposed to those of normal controls, creates a thickening of the thoracolumbar fascia, as well as a reduced difference in thickness in its longitudinal and transverse axes. Normally the longitudinal axis of the thoracolumbar fascia should be more rigid, as it works as a tendon connecting different body segments and different muscles, and the transverse axis of the thoracolumbar fascia should be more adaptable. This allows the thoracolumbar fascia to have a good adaptive capacity that is different in multiple directions of movement. However when the longitudinal and transverse axis of the thoracolumbar fascia become thickened, denser and more fibrotic it loses its anisotropic behaviour creating what the authors described as a “frozen back” (Pirri et al 2023).
Altered movement patterns creating repetitive stresses remodels the thoracolumbar fascia over time. Adhesions that develop between thoracolumbar fascia layers and epimysium of the erector spinae and multifidus, alters the thoracolumbar fascia’s function in transmitting myofascial forces which modifies proprioceptive input (Pirri et al 2023).
This repeated microinjury and inflammation influences nociceptor activation. The resultant mechanosensivity of nociceptive nerves when the thoracolumbar fascia is strained also alters body movement patterns and maladaptive tissue remodelling (Pirri et al 2023).
Mitochondria & sensory processing
Function of mitochondria
Mitochondria regulate inflammatory responses and neuronal functions. Having a higher energetic demand in comparison to other cell types neurones rely heavily on glucose metabolism and fatty acids in mitochondrial respiration to synthesise ATP. This is especially so for primary afferent dorsal root ganglion neurones that have long processes and therefore a high protein turnover which is required for molecules to be produced and transported by axoplasmic flow in sufficient quantities over long distances. Despite the cell bodies of the DRG neurones being large, the majority of the mitochondria are located along the axonal processes of these neurones, although the axon hillock, that, along with the axonal initial segment, determines the passage of action potentials to the central terminals of the sensory neurones in the dorsal horn, possesses larger mitochondria. Whilst mitochondria increase with cell size during development they subsequently decrease with age and can change size and morphology according to the energy demand (Haberberger et al 2023).
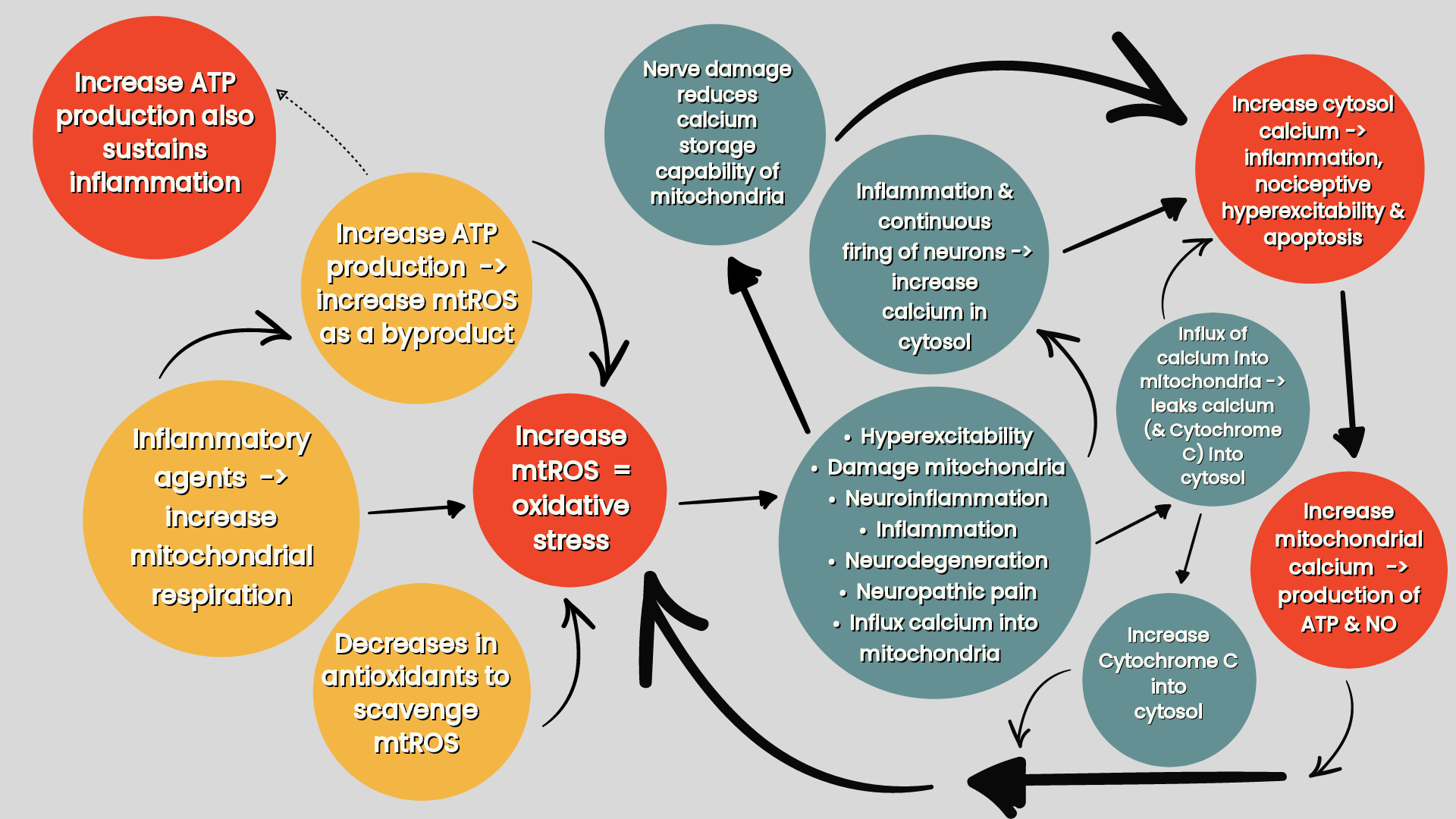
Mitochondrial reactive oxygen species (mtROS) is produced as a byproduct of respiration when producing ATP (Silva et al 2022). Ninety eight percent of inhaled oxygen is utilised by the mitochondria all over the body and one to two percent of total daily oxygen consumption goes to producing reactive oxygen species (ROS) (Kausar et al 2018). Ninety percent of this cellular reactive oxygen species (ROS) being generated by the mitochondria (Silva et al 2022).
MtROS is important for cell signalling and innate host defence against pathogens by initiating inflammatory responses e.g. inflammasome (NLRP3) (Silwal et al 2020). However, when left unchecked, from either (i) ROS overproduction (e.g. increases mitochondrial concentrations of calcium, refer below, or exposure to certain inflammatory agents), or (ii) decreased antioxidant defence, oxidative stress ensues causing damage to the mitochondria, neuroinflammation & neurodegeneration (Silva et al 2022).
Mitochondria (along with the endoplasmic reticulum) also serves as intracellular calcium reservoirs as they regulate intracellular calcium concentration. Under normal physiological conditions the mitochondria should take up more calcium than it releases. This is because calcium is a key regulator of mitochondrial function and stimulates ATP synthesis allowing the mitochondria to produce higher ATP outputs to meet the cellular ATP demands. However, in pathology, high mitochondrial calcium concentrations, along with the accumulation of oxidants and the depletion of adenine nucleotides, makes the mitochondria more porus to molecules. This leads to (i) mitochondrial swelling and death, (ii) stimulates production of mtROS leading to oxidative stress and (iii) releases Cytochrome C from the mitochondria resulting in apoptosis (Brookes et al 2004). It is this extracellular release of Cytochrome C, mtROS or mitochondrial DNA that triggers the inflammatory response that regulates neuronal activity and pain. This is how drugs that inhibit mitochondrial calcium uptake in the spinal cord and neurones reduces pain (Silva et al 2022).
Mitochondria also controls the release of neurotransmitters, neuronal excitability, signalling and plasticity which is also another way through which modulating mitochondrial functions in sensory neurones can reduce hyperalgesia (Silva et al 2022).
Inflammation & mitochondrial respiration
Exposure of neurones to inflammatory mediators alter mitochondrial respiration in neurones. Some inflammatory agents promote mitochondrial respiration which increases ATP production, but as a byproduct of this, produces mtROS which damages the mitochondria and promotes neuroinflammation and neurodegeneration. Increases in ATP production can sustain inflammatory responses (refer ‘figure two’). Other inflammatory mediators reduce mitochondrial respiration which decreases ATP production (refer ‘figure three’) (Silva et al 2022).
Figure two: the effects of inflammatory agents on increasing oxidative stress in the mitochondria (Silva Santos Ribeiro P, Willemen HLDM, Eijkelkamp N 2023. Mitochondria and sensory processing in inflammatory and neuropathic pain).
Figure three: the effects of inflammation on mitochondrial deficiency effecting the lactate/pyruvate ratio (increasing lactate) and the NAD+/NADH ratio (increasing NADH) (Silva Santos Ribeiro P, Willemen HLDM, Eijkelkamp N 2023. Mitochondria and sensory processing in inflammatory and neuropathic pain).
Mitochondrial mitophagy & biogenesis
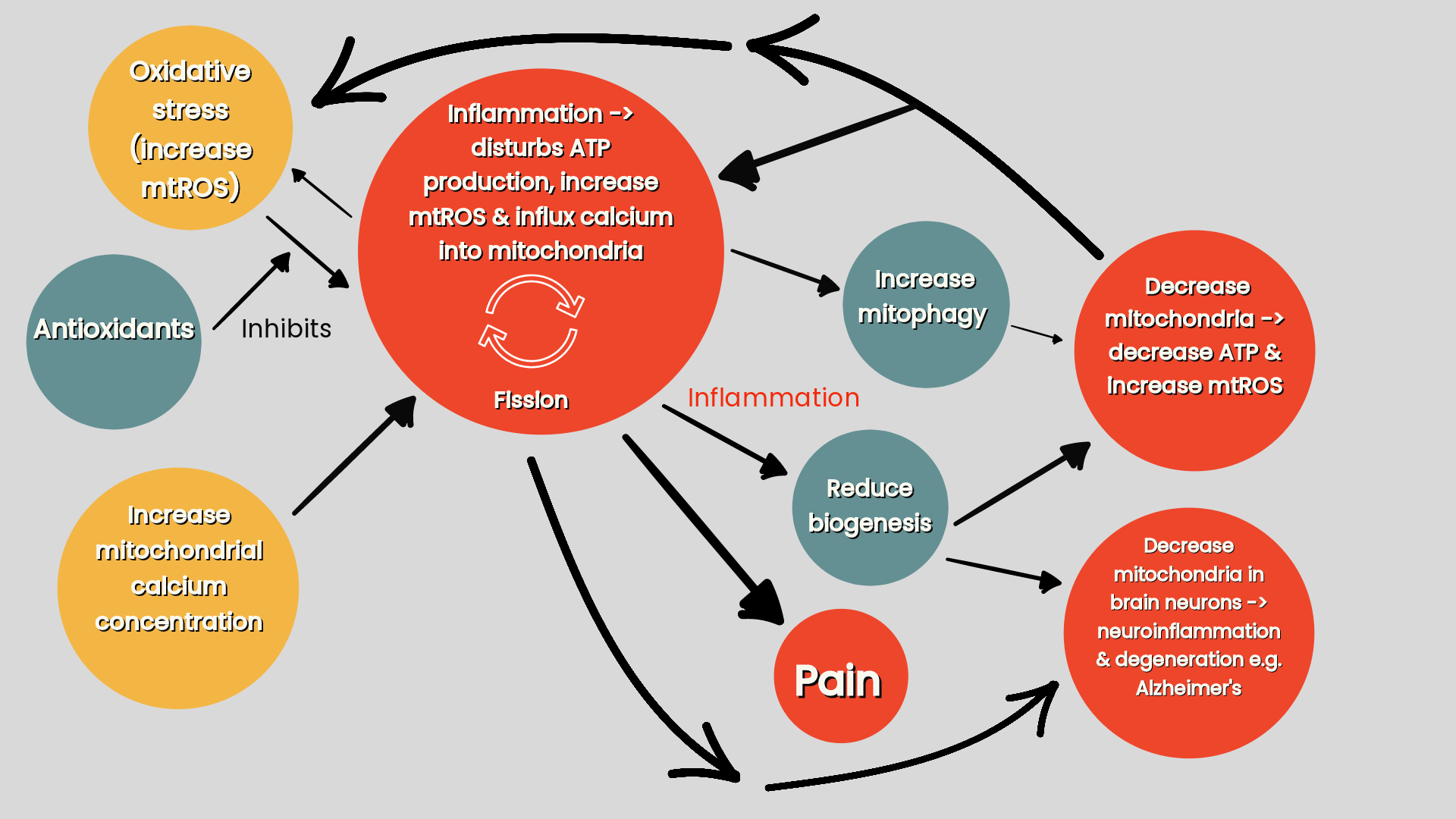
The balance of mitochondrial mitophagy and biogenesis is critical for maintaining proper cellular functions. Biogenesis is driven by decreased energy (ATP) production (Silva et al 2022), oxidative damage to the mitochondria from mtROS (that is a by-product of ATP production) and endurance training and low temperature (4deg C). In response to the need for ATP, and mitochondrial repair from oxidative stress, biogenesis produces new functional mitochondria by triggering their autoreplication from pre-existing mitochondria (Jornayvaz & Shulman 2010).
Consequentially, increase mtROS production or mitochondrial calcium concentration leads to an inflammatory response which leads to the elimination of damaged mitochondria via mitophagy leaving only healthy mitochondria restoring mtROS levels and calcium concentration. Dysfunctional (increased) mitophagy, or defective (decreased) mitochondrial biogenesis affects sensory processing by producing an overall less efficient mitochondrial pool (Silva et al 2022).
Inflammation triggers mitography and inhibits biogenesis, resulting in more mitochondria being destroyed than being produced leading to an overall depletion of mitochondria and impaired ATP and increased mtROS production. This can account for pain in inflammatory conditions, such as osteoarthritis and neuropathic pain, that trigger dysfunctional mitophagy and biogenesis. Decreased mitochondrial biogenesis also reduces the total pool of mitochondria in brain neurons leading to neuroinflammation and neurodegeneration as seen in Parkinson's and Alzheimer's disease (Silva et al 2022).
Mitochondrial fusion and fission
Mitochondrial fusion is when adjacent mitochondria merge permitting a mixture of mitochondrial content it is required for embryonic development and for cell survival at later stages in development. Mitochondrial fusion generally promotes a more efficient and interconnected mitochondrial network as in response to low levels of oxidative stress DNA mutations in one mitochondria can fuse, and be compensated for with DNA from another mitochondria, thereby rescuing the impaired mitochondria. This helps maximise the new mitochondria’s oxidative capacity and in turn its ATP production under stressful conditions (Youle & Bilek 2016).
However, under high levels of oxidative stress badly damaged mitochondria will contaminate other mitochondria if they are allowed to rejoin the mitochondrial network. In such a case mitochondrial fission segregates the damaged parts of mitochondria and targets them for elimination by mitophagy. Therefore, mitochondrial fission by sifting out damaged parts of mitochondria and targeting them for elimination is essential for mitochondrial mitophagy (Youle & Bilek 2016) and biogenesis (Silva et al 2022) in order to create an efficient mitochondrial pool.
Inflammatory conditions, by disturbing ATP production, promoting oxidative stress and calcium influx into the mitochondria, are more likely to induce mitochondrial fission resulting in fragmented mitochondria, impaired energy production, and disrupted mitochondrial homeostasis. In turn, changes in mitochondrial function from mitochondrial fission has a neurotoxic effect, as seen in Alzheimer’s, and promotes the release of inflammatory mediators that sensitise or activate the nervous system contributing to pain (Silva et al 2022) (refer ‘figure four’). Mitochondrial fusion can attempt to compensate for the inflammatory induced breaking up of the mitochondria that triggers mitophagy to eliminate the damaged mitochondria (Youle & Bilek 2016).
Figure four: Inflammation & mitochondrial fission (Silva Santos Ribeiro P, Willemen HLDM, Eijkelkamp N 2023. Mitochondria and sensory processing in inflammatory and neuropathic pain).
Metabolic dysfunction in fibromyalgia & RA
Markers of mitochondrial dysfunction and mitochondrial related genetic modifications are present in rheumatic patients, for instance, RA and fibromyalgia patients express reduced intracellular (e.g. blood, skin) ATP levels and elevated mtROS levels (Silva et al 2022).
References
Macionis V. (2023). Chronic pain and local pain in usually painless conditions including neuroma may be due to compressive proximal neural lesion.
Pirri C, Pirri N, Guidolin D, Macchi V, Porzionato A, De Caro R, Stecco C. (2023). Ultrasound Imaging of Thoracolumbar Fascia Thickness: Chronic Non-Specific Lower Back Pain versus Healthy Subjects; A Sign of a "Frozen Back"?
Kwak HH, Ko SJ, Jung HS, Park HD, Chung IH, Kim HJ (2003). Topographic anatomy of the deep temporal nerves, with references to the superior head of lateral pterygoid.
Teixeira MJ (2016). Concept of acute neuropathic pain. The role of nervi nervorum in the distinction between acute nociceptive and neuropathic pain
Wiberg A, Ng M, Schmid AB, Smillie RW, Baskozos G, Holmes MV, Künnapuu K, Mägi R, Bennett DL, Furniss D. (2019) A genome-wide association analysis identifies 16 novel susceptibility loci for carpal tunnel syndrome.
Gaughran G (1957). FASCIAE OF THE MASTICATOR SPACE
BARKER B & DAVIES P (1972). THE APPLIED ANATOMY OF THE PTERYGOMANDIBULAR SPACE
Topp KS & Boyd BS (2006). Structure and biomechanics of peripheral nerves: nerve responses to physical stresses and implications for physical therapist practice.
Kitamura T, Takagi K, Yamaga M, Morisawa K. (1995). Brachial plexus stretching injuries: microcirculation of the brachial plexus.
Chwei-Chin Chuang D, Fang F, Nai-Jen Chang T, Chuieng-Yi Lu J. (2016). Thoracic Outlet Syndrome: Past and Present-88 Surgeries in 30 Years at Chang Gung.
Silva Santos Ribeiro P, Willemen HLDM, Eijkelkamp N. (2022). Mitochondria and sensory processing in inflammatory and neuropathic pain.
Menovsky, T. (1999). Laser-assisted nerve repair. An experimental study
Baselgia LT, Bennett DL, Silbiger RM, Schmid AB (2017). Negative Neurodynamic Tests Do Not Exclude Neural Dysfunction in Patients With Entrapment Neuropathies
Li Z, Jiang Z, Lu L, Liu Y. (2023). Microfluidic Manipulation for Biomedical Applications in the Central and Peripheral Nervous Systems.
Bove GM. (2008). Epi-perineurial anatomy, innervation, and axonal nociceptive mechanisms.
Foran IM, Hussey V, Patel RA, Sung J, Shah SB. (2018). Native paraneurial tissue and paraneurial adhesions alter nerve strain distribution in rat sciatic nerves
Silwal P, Kim JK, Kim YJ, Jo EK (2020). Mitochondrial Reactive Oxygen Species: Double-Edged Weapon in Host Defense and Pathological Inflammation During Infection
Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. (2004). Calcium, ATP, and ROS: a mitochondrial love-hate triangle
Kausar S, Wang F, Cui H. (2018). The Role of Mitochondria in Reactive Oxygen Species Generation and Its Implications for Neurodegenerative Diseases
Del-Blanco-Muñiz JA, Martín-Vera D, Sosa-Reina MD, Trinidad-Morales A, de-la-Plaza-San-Frutos M, Sánchez-Sierra A. Cervical Impairments in Subjects with Chronic Migraine: An Observational Study
Wu G, Ringkamp M, Murinson BB, Pogatzki EM, Hartke TV, Weerahandi HM, Campbell JN, Griffin JW, Meyer RA. (2002). Degeneration of myelinated efferent fibers induces spontaneous activity in uninjured C-fiber afferents
Jornayvaz FR, Shulman GI. (2010) Regulation of mitochondrial biogenesis
Youle RJ, van der Bliek AM. (2016). Mitochondrial fission, fusion, and stress
Andreeva D, Murashova L, Burzak N, Dyachuk V. (2022). Satellite Glial Cells: Morphology, functional heterogeneity, and role in pain
Krock E, Morado-Urbina CE, Menezes J, Hunt MA, Sandström A, Kadetoff D, Tour J, Verma V, Kultima K, Haglund L, Meloto CB, Diatchenko L, Kosek E, Svensson CI. (2023). Fibromyalgia patients with elevated levels of anti-satellite glia cell immunoglobulin G antibodies present with more severe symptoms.
Bogduk N (1981). An anatomical basis for the Neck-Tongue Syndrome
Haberberger RV, Barry C, Dominguez N, Matusica D. (2019). Human Dorsal Root Ganglia.
Haberberger RV, Kuramatilake J, Barry CM, Matusica D. (2023). Ultrastructure of dorsal root ganglia.
Weerasuriya A & Mizisin A (2011). The Blood-Nerve Barrier: Structure and Functional Significance
Berciano J (2018). Axonal pathology in early stages of Guillain-Barré syndrome.
Aliyarbayova A, Mehraliyeva G, Sadiqova G, Nacafova T, Mansimov A (2022). Ultrastructural peculiarities of perineurial cells of capsular elements of dorsal root ganglia. Animal model of study
Martin SL, Reid AJ, Verkhratsky A, Magnaghi V, Faroni A. (2019). Gene expression changes in dorsal root ganglia following peripheral nerve injury: roles in inflammation, cell death and nociception.
Fanton S, Menezes J, Krock E, Sandström A, Tour J, Sandor K, Jurczak A, Hunt M, Baharpoor A, Kadetoff D, Jensen KB, Fransson P, Ellerbrock I, Sitnikov R, Svensson CI, Kosek E. (2023). Anti-satellite glia cell IgG antibodies in fibromyalgia patients are related to symptom severity and to metabolite concentrations in thalamus and rostral anterior cingulate cortex